Background: In pediatric acute myeloid leukemia (AML), measurable residual disease (MRD) by flow cytometry (FCM) after induction therapy has emerged as one of the strongest prognostic factors. We designed a phase III study (NOPHO-DBH AML2012) using an intensified response-guided induction with mainly MRD based risk stratification. All high-risk patients were treated with allogeneic stem cell transplantation (SCT). The study contained two randomized questions: 1) a randomized comparison of mitoxantrone and liposomal daunorubicin (DNX) in first induction which is reported elsewhere and 2) the comparison of AD(x)E (low dose cytarabine, DNX, etoposide) and FLAD(x) (fludarabine, high dose cytarabine, DNX [60 mg/m 2 x III]) which was proven effective in refractory/relapsed AML in the second induction course
Patients and methods: The study was population-based and conducted in the Nordic countries, Belgium, Hong Kong and the Netherlands (NOPHO-DBH collaboration). Between March 2013 and November 2017, 194 were randomized 1:1 to AD(x)E or FLAD(x). At this time DNX became unavailable and randomization was paused. After almost 18 months, when it became clear that the shortage was permanent, randomization was resumed (February 2019-July 2021) but with daunorubicin substituting DNX. In total 306 patients were included (152 AD(x)E, 154 FLAD(x)). Randomization was stratified according to treatment arm and response to course 1. The primary endpoint was the proportion with MRD <0.1% after the second induction. Patients with MRD ≥ 0.1% after course 2 or MRD ≥ 15% after course 1 or with FLT3-ITD and NPM1 wild type were assigned high-risk (HR) and consolidated with SCT whereas standard-risk (SR) received 3 chemotherapy courses ( CBFB:: MYH11 2 courses) (Fig 1). Estimates of overall survival (OS) and event-free survival (EFS) from date of diagnosis and given at 5 years were calculated by the Kaplan-Meier method and given as percentage± standard error with differences tested by log rank test.
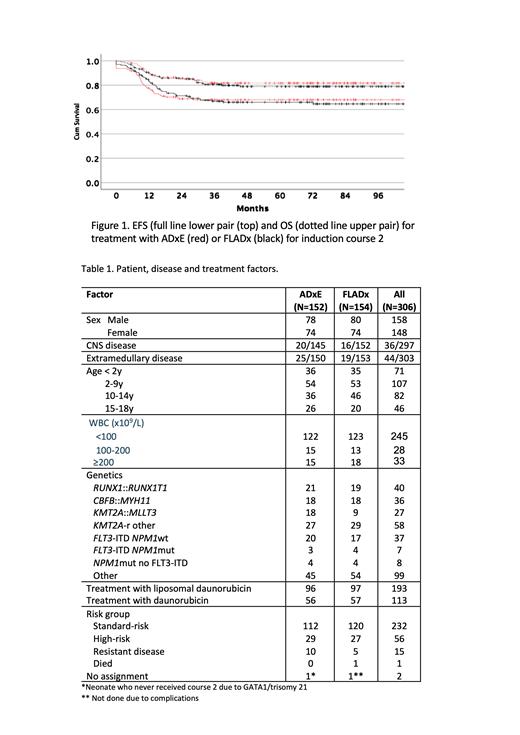
Results: Patient and disease characteristics were similar between treatment arms (Table 1). For all 306 randomized patients EFS was 66.8±2.8% and OS 80.5±2.4%. 232 (76%) had SR with EFS of 71.1±3.1% and OS 86.9±2.3% whereas 56 (18%) had HR with EFS 67.5±6.3% and OS 70.1±6.3%. Median observation time for patients without events was 68 months. There was no difference in proportion of patients with MRD < 0.1% in the randomized groups with 112/152 (74%) for AD(x)E and 120/154 (78%) for FLAD(x). CR rates were 93.4% for AD(x)E and 96.1% for FLAD(x). Survival outcomes for AD(x)E and FLAD(x) were very similar with EFS 67.9±3.8 vs 65.9±3.9 ( P=.93) and OS 82.0±3.2 vs 79.1±3.4 ( P=.60) (fig 1). Univariate Cox regression on all factors in table 1 showed statistical significance for RUNX1:: RUNX1T1 associated with better EFS (Hazard ratio[HzR] 0.26 CI 0.10-0.70) and “other” aberrations with worse EFS (HzR 2.21 CI 1.49-3.27). For OS, both RUNX1:: RUNX1T1 (HzR 0.12 CI 0.02-0.84) and CBFB:: MYH11 (HzR 0.12 CI 0.02-0.88) showed better outcome while the group “other” aberrations had increased HzR (1.77 CI 1.03-3.0). In multivariable analysis, RUNX1:: RUNX1T1 and CBFB:: MYH11 were the only factors significantly associated with EFS and OS (lower HzR) . One patient in each arm died from toxicity after course 2. There was a trend for increased toxicity in patients treated with FLAD(x), with 134 grade 3 (CTCAE4.0) toxicities registered after course 2 compared to 103 for AD(x)E and 37 vs 27 grade 4 toxicities. Also, 18 patients in the FLAD(x) group required ICU treatment compared to 9 for AD(x)E.
Conclusion. Risk stratification based on MRD FCM and response-guided induction as used in the NOPHO-DBH AML 2012 protocol resulted in one of the best cure rates reported in newly diagnosed pediatric AML. The stratification allowed 75% of patients to receive standard-risk treatment without SCT in primary therapy. Patient with suboptimal response (ie our HR group) benefitted from SCT in first remission. Perhaps surprisingly, given the efficacy in relapsed AML, FLAD(x) did not improve outcome compared to AD(x)E. Since toxicity was higher we have opted to retain ADE in the upcoming protocol.
OffLabel Disclosure:
Tierens:BD Biosciences: Honoraria, Speakers Bureau.
The study treated children with liposomal daunorubicin which did not have pediatric AML as registered indication but had been tested in several large clinical trials in de novo as well as relapsed pediatric AML

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal